

Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. It isn't easy, but regular practice can help you achieve perfection in this particular topic.

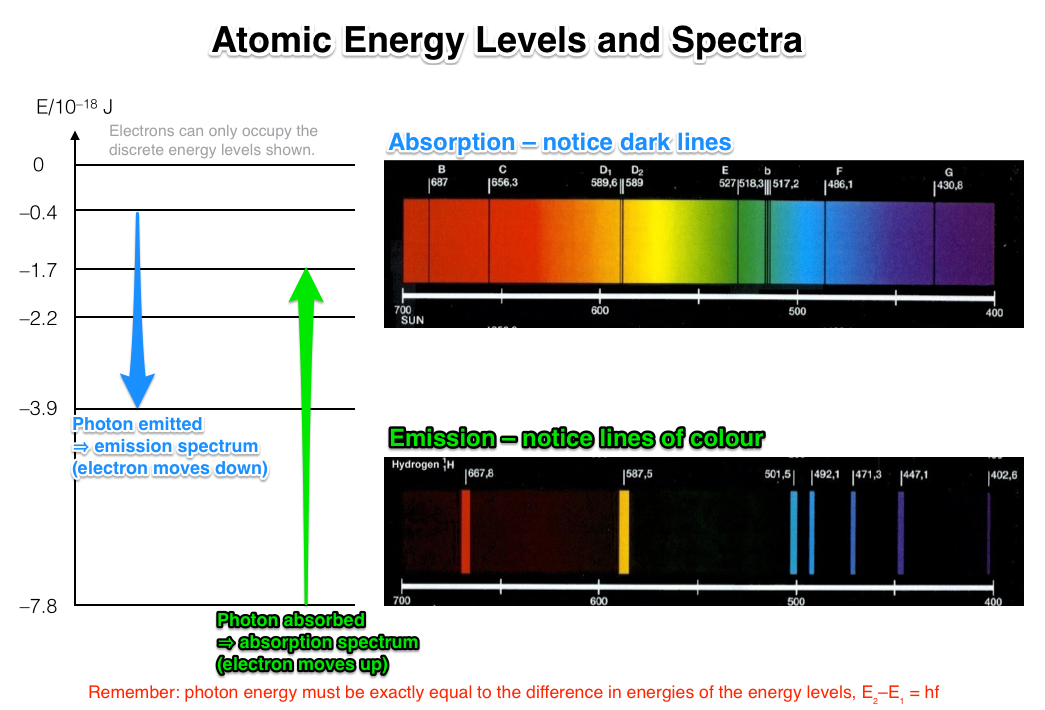
This is what you will learn from the absorption spectrum. Since the light which got re-emitted is unlikely to get emitted in the same direction as one of the absorbed photons, this phenomenon is the reason for dark lines getting created in the spectrum.Īn absorption spectrum gets created from the frequencies of light that are transmitted through dark bands when energy gets absorbed with the help of electrons at the time when they are present in the ground state to reach high energy states. Atoms that are present in the gas starting absorbing in different frequencies. The absorption spectrum is defined as an electromagnetic spectrum within which a decrease in the intensity of radiation at particular wavelengths or various wavelengths characteristic of a specific absorbing substance gets manifested in the form of dark lines or bands.Īn absorption spectrum generates when a light goes through a cold, dilute gas. The above explanation and example can make you understand what absorption is and how it operates using a laser. On the other hand, IR grade fused silica is known for having a lower amount of hydroxide ions this immediately results in greater transmission throughout the NIR spectrum. Still, they experience dips in transmittance, which are centred at 1.4um, 2.2um, and 2.7um due to absorption from Hydroxide ion impurities. The main cause of fluorescence is the impurities that are present in the substrate, for example, rare-earth ions.įor example, UV grade fused silica is a substance that can explain high transmittance in the UV and visible spectra. Most of the time, fluorescence is isotropic and tends to radiate in every direction, which makes things worse than before. Unintentional fluorescence is the reason for loss of energy and also acts as a barrier in signal detection, which is considered detrimental in the case of laser optics applications. These atoms, after this phenomenon, start to fluoresce and begin to emit radiation in the form of photons through spontaneous emission when electrons start falling back to a lower energy level. Electrons that are present in the discrete energy tend to level up the atoms that forced the optical medium to absorb radiative photons and are pushed to the semi-table, higher energy levels. Absorption in Laser PhysicsĪccording to researchers, the laser can get easily absorbed inside an optical substrate by using several methods which are distinct from each other. So, the white strip drawn by you will be less hot as compared to other surfaces that are painted black. If you draw a white line on the black pavement, then it will reflect more because the white colour doesn't absorb more light waves. The black pavement usually becomes hot instantly because it absorbs most of the light waves that reflect on it, and a little of the light waves are reflected back thus, the pavement appears black. Black pavement can be considered as one of the examples because black pavement absorbs energy from light. As there is minimal energy present in the wave, therefore less of the energy gets reflected. Some of the energy of the wave gets reduced during this process because the vibration takes away some of the wave's energy. Absorb PhysicsĪbsorb definition physics is a phenomenon that happens when a wave comes in contact with a medium and forces the molecules of the medium to vibrate, move and change their places. According to wave motion, absorption is considered as the transfer of energy of a wave to a matter this happens when the wave passes through the matter. The absorption of waves never depends on the intensity of the matter. One example of such an absorber is thermal energy. Infrared spectrum of ethanol.According to physics, absorption of electromagnetic radiation is considered as a process that shows how a matter can take up a photon's energy and then transform electromagnetic energy into internal energy of the absorber.


 0 kommentar(er)
0 kommentar(er)
